Outcomes after Early Treatment with Hydroxychloroquine and Azithromycin: An Analysis of 30,423 COVID-19 patients
By Peter A. McCullough, MD, MPH | Courageous Discourse | November 6, 2023
We perform prospective, randomized, double-blind, placebo-controlled trials to test drugs, vaccines, devices, and other products for safety and efficacy. Randomization is important since it handles: 1) selection bias, 2) all known and unknown confounders. Despite the hundreds of billions of dollars spent during the pandemic, we did not have an investment in large, multidrug prospective, randomized, placebo controlled trials or comparative studies to test the best drug regimens.
In the end, what patients care about is how they feel, function, and survive. When it came to COVID-19, whether randomized or not, if patients survived if they were in the optimally treated group. The only way to assess how a high-risk population fared in the pandemic is to report on a large sample of patients sick with COVID-19 with a large number of the outcome of of interest—death.
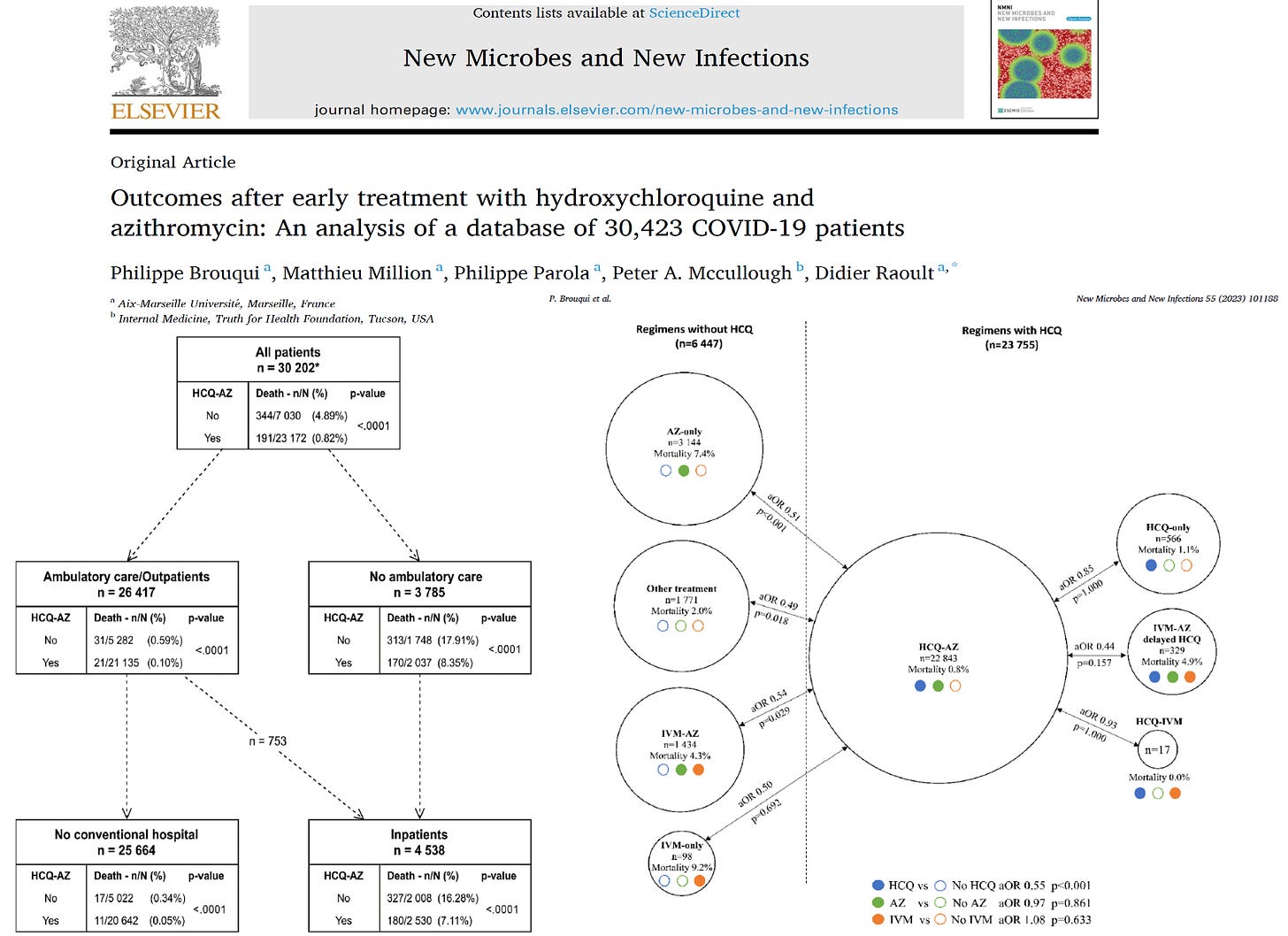
Brouqui et al reported from a French database of 30,423 COVID-19 patients of whom 535 succumbed to the illness. In great detail, the investigators report mortality according to ambulatory treatment received, hospitalization, and the course over the following six weeks.
https://doi.org/10.1016/j.nmni.2023.101188
As you can see, the most favored group was those who received the regimen of hydroxychloroquine and azithromycin early in the course of illness. Of the 30,202 patients for whom treatment information was available, 191/23,172 patients (0.82%) treated with HCQ-AZM died, compared to 344/7,030 patients (4.89%) who did not receive HCQ-AZM. All the other combinations received are reported in the figure.
Important points:
- HCQ+AZM consistently reduced the risk of hospitalization and death
- If hospitalized, those pre-treated with HCQ+AZM at home had a greater chance of survival
Critics say this was not a randomized trial. Patients say it does not matter, they just want to survive on HCQ + AZM! When the differences are this large, we go with what is working for patients, not a false narrative from the Bio-Pharmaceutical Complex deceiving the population on simple, safe, generic drugs.
Share this:
Related
November 6, 2023 - Posted by aletho | Science and Pseudo-Science | Covid-19
No comments yet.
This site uses Akismet to reduce spam. Learn how your comment data is processed.
Featured Video
Ian Proud: Economic Reset with Russia to Save Europe
or go to
Aletho News Archives – Video-Images
From the Archives
Jack Ruby: Israel’s Smoking Gun

BY LAURENT GUYÉNOT • UNZ REVIEW • NOVEMBER 13, 2021
By a strange paradox, most Kennedy researchers who believe that Oswald was “just a patsy” spend an awful lot of time exploring his biography. This is about as useful as investigating Osama bin Laden for solving 9/11. Any serious quest for the real assassins of JFK should start by investigating the man who shot Oswald at pointblank in the stomach at 11:21 a.m. on September 24, 1963 in the Dallas Police station, thereby sealing the possibility that a judicial inquiry would draw attention to the inconsistencies of the charge against him, and perhaps expose the real perpetrators. One would normally expect the Dallas strip-club owner Jack Ruby to be the most investigated character by Kennedy truthers. But that is not the case. … continue
Blog Roll
-
Join 2,405 other subscribers
Visits Since December 2009
- 7,381,312 hits
Looking for something?
Archives
Calendar
Categories
Aletho News Civil Liberties Corruption Deception Economics Environmentalism Ethnic Cleansing, Racism, Zionism Fake News False Flag Terrorism Full Spectrum Dominance Illegal Occupation Mainstream Media, Warmongering Malthusian Ideology, Phony Scarcity Militarism Progressive Hypocrite Russophobia Science and Pseudo-Science Solidarity and Activism Subjugation - Torture Supremacism, Social Darwinism Timeless or most popular Video War Crimes Wars for IsraelTags
9/11 Afghanistan Africa al-Qaeda Australia BBC Benjamin Netanyahu Brazil Canada CDC Central Intelligence Agency China CIA CNN Covid-19 COVID-19 Vaccine Donald Trump Egypt European Union Facebook FBI FDA France Gaza Germany Google Hamas Hebron Hezbollah Hillary Clinton Human rights Hungary India Iran Iraq ISIS Israel Israeli settlement Japan Jerusalem Joe Biden Korea Latin America Lebanon Libya Middle East National Security Agency NATO New York Times North Korea NSA Obama Pakistan Palestine Poland Qatar Russia Sanctions against Iran Saudi Arabia Syria The Guardian Turkey Twitter UAE UK Ukraine United Nations United States USA Venezuela Washington Post West Bank WHO Yemen ZionismRecent Comments
 Aletho News
Aletho News- Ian Proud: Economic Reset with Russia to Save Europe
- Senator Rand Paul Introduces Federal Bill to END Vaccine Makers’ Liability Shield
- The Hidden Map: US and Israel May Use Unexpected Neighbors to Attack Iran
- Trump, Netanyahu Agree to Target Iranian Oil Exports to China
- Russia open to discussing Ukraine’s ‘external governance’ – senior diplomat
- US Caribbean Buildup Near $3B — Report
- Munich Security Conference and the U.S. elephant in the room
- Epstein Pitched JPMorgan Chase on Plan to Get Bill Gates ‘More Money for Vaccines’
- Germany’s CDU Pushes Real-Name Social Media Mandate and ID Checks
- Patrik Baab: Europe’s New Iron Curtain – Freedom of Speech Dies
 If Americans Knew
If Americans Knew- Israel-backed border guards, GHF-linked aid – Not a ceasefire Day 128
- Israel battles Palestinian right of return, one Palestinian at a time – Not a ceasefire Day 127
- Noor’s short life of unimaginable suffering
- Israel Destroyed Gaza’s Hospitals. Now It’s Banning Doctors Without Borders.
- Is Spite of What Zionists Say, It’s a Good Thing to Criticize Governments
- Palestinian mother, daughter recount strip searches, harsh conditions in Israeli detention
- Israel used weapons in Gaza that made thousands of Palestinians evaporate
- ADL’s Stats Twist Israel’s Critics Into Antisemites
- Why Is the World Silent When the Gaza Genocide Is Not Over?
- In Gaza: 8,000 bodies under rubble, 3,000 missing – Not a ceasefire Day 126
 No Tricks Zone
No Tricks Zone- Unfudging The Data: Dutch Meteorological Institute Reinstates Early 20th Centruy Heat Waves It Had Erased Earlier
- German Gas Crisis…Chancellor Merz Allegedly Bans Gas Debate Ahead of Elections!
- Pollen Reconstructions Show The Last Glacial’s Warming Events Were Global, 10x Greater Than Modern
- Germany’s Natural Gas Storage Level Dwindles To Just 28%… Increasingly Critical
- New Study Rebuts The Assumption That Anthropogenic CO2 Molecules Have ‘Special’ Properties
- Climate Scientist Who Predicted End Of “Heavy Frost And Snow” Now Refuses Media Inquiries
- Polar Bear Numbers Rising And Health Improving In Areas With The Most Rapid Sea Ice Decline
- One Reason Only For Germany’s Heating Gas Crisis: Its Hardcore-Dumbass Energy Policy
- 130 Years Later: The CO2 Greenhouse Effect Is Still Only An Imaginary-World Thought Experiment
- New Study Affirms Rising CO2’s Greening Impact Across India – A Region With No Net Warming In 75 Years
Contact:
atheonews (at) gmail.com
Disclaimer
This site is provided as a research and reference tool. Although we make every reasonable effort to ensure that the information and data provided at this site are useful, accurate, and current, we cannot guarantee that the information and data provided here will be error-free. By using this site, you assume all responsibility for and risk arising from your use of and reliance upon the contents of this site.
This site and the information available through it do not, and are not intended to constitute legal advice. Should you require legal advice, you should consult your own attorney.
Nothing within this site or linked to by this site constitutes investment advice or medical advice.
Materials accessible from or added to this site by third parties, such as comments posted, are strictly the responsibility of the third party who added such materials or made them accessible and we neither endorse nor undertake to control, monitor, edit or assume responsibility for any such third-party material.
The posting of stories, commentaries, reports, documents and links (embedded or otherwise) on this site does not in any way, shape or form, implied or otherwise, necessarily express or suggest endorsement or support of any of such posted material or parts therein.
The word “alleged” is deemed to occur before the word “fraud.” Since the rule of law still applies. To peasants, at least.
Fair Use
This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of environmental, political, human rights, economic, democracy, scientific, and social justice issues, etc. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. For more info go to: http://www.law.cornell.edu/uscode/17/107.shtml. If you wish to use copyrighted material from this site for purposes of your own that go beyond ‘fair use’, you must obtain permission from the copyright owner.
DMCA Contact
This is information for anyone that wishes to challenge our “fair use” of copyrighted material.
If you are a legal copyright holder or a designated agent for such and you believe that content residing on or accessible through our website infringes a copyright and falls outside the boundaries of “Fair Use”, please send a notice of infringement by contacting atheonews@gmail.com.
We will respond and take necessary action immediately.
If notice is given of an alleged copyright violation we will act expeditiously to remove or disable access to the material(s) in question.
All 3rd party material posted on this website is copyright the respective owners / authors. Aletho News makes no claim of copyright on such material.

Leave a comment