Hepatitis B Vaccination of Newborns: Seriously Misleading Media Reports
By Peter C. Gøtzsche | Brownstone Institute | December 19, 2025
Fiction or faith. It is a major failure to give equal prominence to people presenting scientific facts and people talking about their feelings or beliefs with no evidence in their support, or to allow them to contradict unchallenged the most reliable evidence we have.
However, virtually every time I know something about a healthcare issue considered controversial, this is what I see in the news, and the hepatitis B vaccine controversy illustrates this abundantly.
On 5 December 2025, with a vote of 8 versus 3, the Advisory Committee on Immunization Practices (ACIP) at the Centers for Disease Control and Prevention (CDC) ended the recommendation that all newborns in the United States receive a hepatitis B shot at birth. The birth dose was recommended only if the mother had tested positive for the virus or if her infection status was unknown.
The change was very rational, and as in Western Europe, only Portugal recommends a universal birth dose, it would seem difficult to argue against it. But the media did and failed us badly. Two days after the vote, I downloaded news stories from 14 major media outlets, and they were all very negative. The media used three main tactics to support their beliefs:
They denigrated the Secretary of Health, Robert F. Kennedy, Jr., the members of ACIP he had selected, and some of the presenters at the meeting.
They gave undue prominence and praise to the three dissenting ACIP voices and outsiders, who were depicted as experts or scientists, as if to say that they must be right, and they were widely quoted for their remarks, which were rarely rational or evidence-based.
They didn’t check if what the critics of the policy change claimed was correct.
The Denigration of Kennedy
Of the 14 news outlets, only Nature did not denigrate Kennedy.
Reuters started its press release by saying it was “a major policy win” for Kennedy that vaccine advisers named by him reversed a decades-long recommendation “that disease experts say will reverse decades of public health gains.” So, Kennedy’s advisers were not experts, and as the critics were experts, they must be correct, right?
Reuters noted that the CDC is “now run by a Kennedy-appointed acting head, Jim O’Neill, who is not a scientist;” that Kennedy founded the anti-vaccine group Children’s Health Defense; fired ACIP’s previous 17 “independent” experts and replaced them with a group that largely supports his views; dropped broad recommendations for the Covid vaccine and cut funding for mRNA vaccines.
The facts are that several of the previous experts at the ACIP were not independent but had conflicts of interest in relation to vaccine manufacturers and other drug companies; that recommending Covid vaccines only to high-risk groups brought the US on par with Europe; and that cutting funding for mRNA vaccine research was well motivated. Kennedy said that his team had reviewed the science and found that these vaccines fail to protect effectively against upper respiratory infections like Covid and flu. His department was therefore shifting the funding toward “safer, broader vaccine platforms that remain effective even as viruses mutate.”
Reuters misrepresented the ACIP meeting entirely, claiming that “many of Kennedy’s committee members criticized the vaccine as unsafe.” What they said was that safety had not been adequately studied, which was correct.
The other media called Kennedy a vaccine sceptic (The Hill, Health Policy Watch, CBC), a vaccine activist (CNN, the Guardian), or an anti-vaccine advocate (PBS), who fired all 17 previous members of the ACIP, replacing them with people who largely shared his scepticism (New York Times, Washington Post, National Public Radio, CNN, PBS, CBS News, Time, Health Policy Watch, CBC, BBC, Guardian ) with a “goal of upending vaccine policy” (New York Times ), and the vote fulfilled a long-held goal of the anti-vaccine movement (The Hill ).
The CBC, the largest news broadcaster in Canada, noted that Kennedy had promoted debunked theories linking vaccines to autism. It is correct that studies of the MMR vaccine and aluminium adjuvants did not find a link, but the aluminium study is seriously flawed, some studies have claimed a link, and as it has not been studied if the extensive US childhood vaccine program might cause autism, the CDC has suggested additional research projects.
The Washington Post said that aluminium had become a focal point for anti-vaccine groups that claim cumulative exposure may harm neurological development and that vaccine researchers note that aluminium is present naturally in breast milk, food, and water at far higher levels than in vaccines and is rapidly cleared from the body. It is highly misleading to compare dietary intake with injections, as very little aluminium is absorbed from the gut and the rest is effectively eliminated via the kidneys, and as aluminium adjuvants in vaccines are harmful.
The Hill and CNN noted that aluminium adjuvants in vaccines have been proven to be safe (which is false), but that vaccine sceptics like Kennedy have long said they are linked to allergies and other health conditions (which is correct). Natural infection protects against allergies, and studies comparing vaccinated with unvaccinated children have shown vaccines increase the occurrence of asthma and other atopic diseases.
The Denigration of ACIP Members and Meeting Presenters
Nature noted that several panel members continued to express broad criticism of vaccines.
The New York Times lamented that most of the new ACIP members and some of the presenters have no experience in vaccine research or clinical practice and that the divisiveness and dysfunction of the committee in making the decision raised questions about the reliability of the advisory process.
This is terribly misleading. People who have learned to read can assess the merits of vaccines, and scientific debate is what furthers science. Acting ACIP chair Robert Malone said that the committee’s work must be guided by evidence, transparency, and a willingness to scrutinise assumptions rather than protect them.
Health Policy Watch wrote that Malone has been criticised for vaccine misinformation, which is a meaningless comment without any mention of what the issues were. Some of the most outstanding vaccine researchers in the world, professors Peter Aaby and Christine Stabell Benn from Copenhagen, have been criticised for misinformation and have had lectures and interviews removed from YouTube even though everything they said was correct.
CBS News noted that ACIP member Retsef Levi, a mathematician with no medical training (so what?), had falsely claimed that experts had never tested the vaccines appropriately, and the New York Times called it incorrect when lawyer Aaron Siri, a presenter, said that “not one” of the shots administered to children had been compared against a placebo or an inert substance. But Levi and Siri were correct. No childhood vaccine on CDC’s schedule was studied in placebo-controlled trials or relied upon before licensure.
The CBC also described Levi as a person with no medical degree who had questioned the safety of the Covid-19 vaccines and called for Covid vaccine programs to be halted. Well, I have observed repeatedly that Levi’s arguments were far more persuasive than those offered by people with medical degrees, e.g. by ACIP member Cody Meissner, a paediatric infectious-disease specialist (see below).
And Covid vaccines are definitely not safe; they have killed children who developed myocarditis and adults who developed blood clots. It was very prudent to change the “all-inclusive” US Covid vaccine programs when by far most people have been infected, whether vaccinated or not, and because repeated boosters can weaken the immune system and increase the risk of respiratory infections, also for flu shots. Healthcare workers themselves have already delivered a verdict. According to the CDC’s own data, fewer than 10% received a booster in the past year.
National Public Radio denigrated Siri: an anti-vaccine lawyer with no medical or scientific training, and the Washington Post failed their readers, too: “Aaron Siri, a Kennedy ally and lawyer for the anti-vaccine movement, delivered a presentation for more than 90 minutes. Siri said clinical trials for vaccines have not been properly performed, that safety surveillance after vaccines are licensed is lacking and that the efficacy of vaccines in reducing deaths and spread of disease has been overstated. Siri and Kennedy-aligned activists argue that the cumulative number of shots places an undue burden on child immune systems. Scientists counter that… the immune system can safely handle far more antigens than vaccines contain.”
Siri is correct and the reason why he was given so much time is that he is evidence-based and very knowledgeable. His book about vaccines is outstanding. And “scientists” have no evidence that the immune system can safely handle many vaccine antigens injected simultaneously. This is unknown and needs studying.
The Washington Post also noted that “Siri petitioned the government in 2022 on behalf of the anti-vaccine group Informed Consent Action Network, which is run by Kennedy’s former communications director, to reconsider its approval of Sanofi’s stand-alone polio vaccine. Siri argued that the government had relied on inadequate data, a claim regulators rejected.”
However, the petition notes that “the clinical trials relied upon to license this product did not include a control group and only assessed safety for up to three days after injection. These trials therefore did not comply with the applicable federal statutory and regulatory requirements necessary to prove the product was ‘safe’ prior to licensure.” As live, attenuated polio vaccines can mutate and cause polio, I agree with Siri that this drug had not been adequately studied before licensure.
The New York Times and National Public Radio wrongly implied that Siri wanted to remove all polio vaccines (“polio vaccines” or “the polio vaccine”).
Praising “Experts” and Giving Them Undue Prominence
Safety was a major issue. Dissenting ACIP member Cody Meissner said at the meeting that we know that the vaccine is safe, and his reassurances were quoted by the New York Times, the Washington Post, National Public Radio, Nature, the BBC, and Time.
However, when the Institute of Medicine in 2013 was commissioned to review the safety of the CDC childhood vaccine schedule, they could not find a single study that had compared health outcomes in vaccinated children with those in children who had not received any vaccines and they concluded: “There is no evidence that the schedule is not safe.” Similarly, Time wrote about the hepatitis B vaccine that there is “no evidence in regard to lack of safety.” My comment on this kind of reasoning was: “If the brakes in a new car model have never been tested, the reassuring conclusion would be: ‘There is no evidence that the brakes don’t work.’”
At the ACIP meeting, Meissner accused Siri of presenting “a terrible, terrible distortion of all the facts” (New York Times, National Public Radio, The Hill, CNN, Time ) and of making “absolutely outrageous statements about safety.” This was totally false and Meissner should know better. ACIP members were shown that the clinical trials underpinning approval of the hepatitis B vaccine were small, lacked a placebo group, and followed infants for no more than seven days after vaccination, which would not detect any long-term adverse outcomes. Normally, such findings would have shocked people and prompted caution, but Meissner insisted that “There is no evidence of harm.” Well, if you don’t look, you won’t find.
Levi hit the nail on the head: “What is the number needed to vaccinate – among babies born to hepatitis B-negative mothers – to prevent one case of chronic hepatitis B?” No one supplied an answer. But if the true number was “in the millions,” then any credible harm-benefit analysis would require showing a number-needed-to-harm one infant seriously even higher.
Meissner, however, opined that the move was rooted in baseless scepticism and that we will see more hepatitis B infections (Washington Post, Nature ). He was also against possibly using fewer than three doses of the vaccine (New York Times, The Hill ), arguing that antibody titres are not a good correlate of protection and did not have scientific backing (Nature ). The inconsistency was unmistakable. Antibodies are embraced as proof of vaccine efficacy when convenient, e.g. in drug regulation, otherwise not.
Another dissenting ACIP member, psychiatrist Joseph Hibbeln, was quoted a great deal although he said nothing of substance: The revised guidance was “unconscionable” (Washington Post ), “the decisions should be based on data” (The Hill ), “Those are all speculations” (Time ), “Is there any specific evidence of harm of giving this vaccination before 30 days?” (Guardian ). Not a single journalist wondered why a psychiatrist sat in a vaccine committee.
Dr Tracy Beth Høeg, a presenter at the meeting, noted that the US was an outlier recommending around 72 childhood vaccine doses, while countries like Denmark use fewer than 30. PBS and Time argued that the US is not an outlier in recommending hepatitis B vaccines for newborns because 116 of the 194 WHO member states did the same. This is not a proper comparison, and, as noted above, in Western Europe, only Portugal recommends a universal birth dose.
Levi noted that “The policy in the US is completely misaligned with many countries that… care about their children just as much as we do,” and when Meissner viewed the growth of the childhood vaccine schedule as an achievement for child health, Siri countered correctly that the US “has the worst health outcomes amongst all developed countries.”
The media quoted three previous CDC directors. Rochelle Walensky said that over the past few months, she had observed “a systematic undermining of the nation’s vaccine program” (National Public Radio) and that the “US vaccine-safety monitoring system can detect very, very rare safety events“ (Nature ). Maybe, but she ignored them. In April 2021, cases of myocarditis after Covid-19 vaccination, particularly among young male vaccine recipients, had been reported to the Vaccine Adverse Event Reporting System at the CDC, but Walensky said by the end of the same month: “We have not seen a signal and we’ve actually looked intentionally for the signal in the over 200 million doses we’ve given.”
Tom Frieden provided a doomsday statement: “The ACIP recommendation… puts millions of American children at greater risk of liver damage, cancer and early death.” He advised everyone to “stand up for fact-based care” and “not accept this misguided and dangerous recommendation” (Time).
Demetre Daskalakis had a weird argument: “This will signal to clinicians that there is something wrong with the vaccine – there is not” (Reuters, CNN). It could also signal greater responsibility at the CDC than under previous directors. But the BBC and the Washington Post joined the folly arguing that public health experts, representatives of medical organisations, and some ACIP members worried the vote could raise unfounded safety concerns about the vaccine and undermine hard-won trust in vaccines leading to more sickness.
The media gave organisations undue prominence without ever considering if they were impartial. They urged people to look to “independent recommendations,” e.g. from the American Medical Association and the American Academy of Pediatrics, for “science-based advice” (National Public Radio).
I would call it advice based on money. The Academy would continue to support the birth dose of the vaccine (Reuters, CBS News, Health Policy Watch, CNN, Time, CBC) but all journalists forgot to say that it receives many millions of dollars from vaccine manufacturers and other drug companies. Unsurprisingly, hepatitis B vaccine makers Merck, Sanofi, and GSK defended their products as safe, and Merck was “deeply concerned by the vote” (Reuters ). Perhaps because Merck’s shares dropped?
“Don’t listen to ACIP at all… listen to the American Academy of Pediatrics” (CNN), which said that the “irresponsible and purposely misleading” guidance would harm children; called it a “deliberate strategy to sow fear and distrust among families” (CBC); and delivered a gigantic falsehood: “Vaccine recommendations are largely similar across developed countries” (CBS News).
Reuters noted that ACIP members had said that the birth dose “was out of step with peer countries, particularly Denmark,” but then quoted “a CDC disease expert” for saying that the US is not comparable to Denmark with its universal healthcare and more thorough screening for the virus. The Washington Post said that “public health experts” had noted that European countries recommending fewer shots for children were smaller and had better health care systems, and that medical associations had argued that the US schedule had been thoroughly studied (which is blatantly false). None of the media quoted Levi, who mentioned that the US and Denmark have the same background rate of hepatitis B despite different policies on the birth dose.
The American Medical Association is also heavily corrupted by industry money and said that ACIP’s decision was “reckless and undermines decades of public confidence in a proven, lifesaving vaccine. Today’s action is not based on scientific evidence” (CNN).
The American College of Physicians said that “This vote… will only endanger children and increase risk of death for millions,” and a hepatitis researcher urged people to “go back to our true experts… our CDC colleagues” (Health Policy Watch).
Time noted that “A group of several dozen professional medical organizations and health advocacy groups, including the American Medical Association” expressed alarm over the committee’s decisions: “Previously, we could expect science to drive decisions.”
Some panellists and media noted that universal hepatitis B vaccination at birth had helped to nearly eliminate cases among newborns in the United States, and that there was no evidence of harm (New York Times, Washington Post, The Hill, Guardian ). However, absence of evidence is not evidence of absence. When Levi countered that the risk for a child of getting infected was extremely low, supporters of the birth dose noted that the virus can be spread by household objects like toothbrushes, razors, or combs used by an infected person. This is a fake argument and the CDC website is explicit: “Although HBV can be found in saliva, it is not spread through kissing or sharing utensils. It is also not spread through sneezing, coughing, hugging, breastfeeding, or food or water.”
Levi also said that the decline in hepatitis B cases occurred long before the birth-dose policy was introduced and was concentrated in older age groups, not among infants, which supported a risk-based policy, focused on infants born to hepatitis B-positive mothers and on high-risk adult populations. When ACIP liaison Dr Flor Muñoz of the Infectious Diseases Society of America claimed that much of the discussion amounted to “misinformation,” Levi responded: “It’s not misinformation… this is CDC data.” When Muñoz pushed back, presenting her disagreement as established fact, Levi replied: “I appreciate your beliefs and feelings about this, but these beliefs and feelings are not supported by the data that were presented.”
Levi also pointed to ACIP’s prior recommendation of Covid-19 vaccination for healthy, extremely low-risk children, which he described as “one of the most outrageous” examples of framework failure.
ACIP’s decision sparked anger from Republican Senator Bill Cassidy (R-LA), a doctor, who said the vaccine is safe and effective (BBC, CBS News, Time, Health Policy Watch). He wrote on X that “Siri, a prominent anti-vaccine lawyer, makes his living suing vaccine manufacturers and is presenting as if an expert on childhood vaccines. The ACIP is totally discredited” (Washington Post, The Hill ).
The Hill was particularly critical. It wrote about an ardent objection from major medical organisations, internal spats among ACIP members, and a stark lack of data to support altering decades-long vaccine guidance, in fact, “There’s been great data and studies done on these vaccines, and they are safe and effective.” The Hill quoted top figures from Illinois, Massachusetts, and New York City for their rants, which included that they would not abide by ACIP’s “irresponsible attacks on clear, evidence-based science.”
When journalists “dial-a-quote,” they call organisations or people whom they know will respond in a way that mirrors their own bias pretending they have asked an “independent expert.”
The media were full of evidence-free, derogatory comments that were meaningless because they could not be contested:
- “We can no longer trust federal health authorities when it comes to vaccines,” “heartbreaking to see this science-driven agency turn into an ideological machine” (New York Times );
- “Medical experts have argued that it’s important to vaccinate all newborns for hepatitis B” (Washington Post );
- “The vaccine is incredibly safe,” experts decried the move (Reuters );
- the American Association of Immunologists is “extremely disappointed” in the decision;
- the American College of Physicians called the meeting “completely inappropriate” (CBS News); “many experts expressed dismay at today’s decision” (CNN);
- “A long lineup of medical experts…strongly urged against changing the vaccination schedule” (Health Policy Watch);
- “Public health experts decried the move,” CDC and the ACIP are no longer trustworthy sources and are becoming increasingly irrelevant (CBC);
- “a forum for the discussion of falsehoods,” ACIP members promoted their own sceptical views on vaccines, looking for a bogeyman, and you’re not going to find something if it doesn’t exist (Time );
- “Experts say any change to the current hepatitis B vaccination recommended schedule could have significant and far-reaching consequences for childhood health in the US” (Guardian ).
When the media presented statements that could be contested, they were usually wrong or seriously misleading, e.g. “Siri’s presentation was replete with ‘falsehoods and misrepresentation of the data,’ and he conflated informed consent with mandates” (New York Times ); “fierce objections from medical groups that said the recommendation had proved a successful public health strategy, nearly eradicating the dangerous virus among U.S. children” (Washington Post); a “Minority of members argue the change is not supported by data” (Reuters ).
Persuasion by Big Numbers
Like the drug industry does, the media used big numbers in their propaganda.
Globally, the vaccine has prevented millions of infections (Health Policy Watch). Before the vaccine, around 200,000 to 300,000 people were infected each year; since the vaccines began being universally administered to babies, overall cases are down to around 14,000 annually (PBS).
After a birth dose was recommended in 1991, the shots have prevented an estimated 90,000 deaths in the US (BBC) and reduced hepatitis B infections among infants and children by 99% (CBS News, Time, Health Policy Watch, Nature ).
All these claims are false or seriously misleading. Data presented at the meeting showed that much of the decline in hepatitis B infections over past decades occurred before the birth dose was recommended and it was largely driven by behaviour change, screening, and targeted vaccination of high-risk groups.
Senator Cassidy wrote on X that “Before the birth dose was recommended, 20,000 newborns a year were infected with hepatitis B. Now, it’s fewer than 20” (CBS News, CNN, Health Policy Watch). This was an error of 133 times. CDC data show that in 1990, only around 150 children below one year of age became infected.
Vaccinologist Paul Offit Lied on CNN
The most high-profile vaccinologist in the world, after vaccine “Godfather” Stanley Plotkin, is Paul Offit, but that may be a thing of the past after Siri’s recent revelations and his self-destructing appearance on CNN on the second day of the ACIP meeting.
Offit told viewers he had not been invited to speak at the meeting but internal documents show his claim is false. CDC officials had contacted him repeatedly – via emails, phone calls and a speaker-request form – inviting him to present.
Offit warned viewers that “50% of people in this country have chronic hepatitis B and don’t know it” (only about 0.3% have chronic disease) and suggested newborns were at risk through everyday contact with nannies, daycare workers, and family members because of sharing toothbrushes, towels, or simply being held by an infected adult, which the CDC denied could happen.
Offit described ACIP as a “clown show,” an “anti-vaccine advisory committee” that “puts children in harm’s way.” He lied monstrously saying that before universal infant vaccination, “30,000 children under the age of 10” contracted hepatitis B each year. CDC data presented at the ACIP meeting showed that new hepatitis B cases in children under the age of 10 were around 400 per year before the universal birth dose was introduced.
I am very indebted to journalist Maryanne Demasi, PhD, who wrote many of the articles I quoted above. She gave Offit the opportunity to clarify his remarks but he did not respond. This silence contrasts sharply with the certainty he brings to national television, where his claims are delivered without scrutiny and his financial ties to vaccine manufacturers are almost never mentioned.
Offit is not an impartial commentator. He earned millions from the sale of his stake in Merck’s rotavirus vaccine, RotaTeq, and has long been aligned with the pharmaceutical industry whose products he routinely defends. Yet major news outlets present him as a neutral authority and take his statements at face value.
Conclusions
The media’s reporting on the hepatitis B issue was seriously misleading and their advice that we should trust the “experts” who condemned the ACIP committee’s wise decision is horribly misguided.
The new ACIP’s first chair was biostatistician Martin Kulldorff. He developed the monitoring system the CDC uses for quick detection of vaccine harms, considered the best in the world. On 1 December, Kennedy announced that Kulldorff was appointed to a senior role at the Department of Health and Human Services after he had “transformed ACIP from a rubber stamp into a committee that delivers gold-standard science for the American people.” NIH director Jay Bhattacharya said that “Five years ago, Martin Kulldorff and I co-authored the Great Barrington Declaration calling for an end to pandemic lockdowns. That evidence-based approach to public health now permeates HHS.”
What the media presented was what we call eminence-based medicine, and the medical journals’ reporting on vaccine issues is also a disaster. I shall end with the abstract of an article I published on 10 November:
The reactions to Robert F. Kennedy Jr.’s initiatives to improve vaccine safety have been almost uniformly negative. I studied how the narratives were framed in a cohort of 33 articles in the BMJ of which 30 were written by journalists or the editor. I focused on whether the reporting was balanced and informative, and whether the articles saw any merit in Kennedy’s reforms in his role as Secretary of Health and Human Services or supported the status quo.
The reporting in the BMJ was highly biased. Much of the information provided in Kennedy’s disfavour was misleading, and some was wrong. All initiatives at improving vaccine safety were condemned, without any analysis of their merits in an evidence-based fashion. Instead, the BMJ cited people who had their own agendas and who condemned Kennedy without providing any evidence in their favour while expressing faith in vaccines, with the industry mantra that they are safe and effective, although all drugs will harm some people.
The BMJ did not take any interest in the widespread and lethal corruption in US healthcare institutions – one of Kennedy’s focus points – but toned it down.
Despite the constant ad hominem attacks, Kennedy has succeeded to introduce important changes and plans related to vaccine safety, guidance about how vaccines are used, and about avoiding neurotoxic metals in vaccine adjuvants.
Dr. Peter Gøtzsche co-founded the Cochrane Collaboration, once considered the world’s preeminent independent medical research organization. In 2010 Gøtzsche was named Professor of Clinical Research Design and Analysis at the University of Copenhagen. Gøtzsche has published over 100 papers in the “big five” medical journals (JAMA, Lancet, New England Journal of Medicine, British Medical Journal, and Annals of Internal Medicine). Gøtzsche has also authored books on medical issues including Deadly Medicines and Organized Crime.
Gardasil on Trial: Did Merck Mislead the Public on Cervical Cancer Prevention?
Top expert delivers a damning report accusing Merck of misleading the public about Gardasil’s ability to prevent cervical cancer
By Maryanne Demasi, PhD | February 24, 2025
With the landmark trial against Merck adjourned until September 2025, new evidence suggests the vaccine manufacturer may have deliberately misrepresented the necessity of mass HPV vaccination.
This revelation comes from an expert report by Dr Sin Hang Lee, a pathologist renowned for his expertise in molecular diagnostics. His findings raise serious concerns about Gardasil’s efficacy and the motives behind its aggressive marketing.

Dr Sin Hang Lee, director of Milford Molecular Diagnostics, Connecticut
Does Gardasil Prevent Cervical Cancer?
Since its introduction in 2006, Gardasil has been marketed as a breakthrough in the fight against cervical cancer.
Yet, as Dr Lee bluntly states in his report, “There is no conclusive evidence that Gardasil has prevented a single case of cervical cancer in the past 18 years.”
No randomised controlled trial (RCT)—the gold standard for assessing efficacy—has ever demonstrated that Gardasil prevents cervical cancer.
Instead, Merck relied on surrogate markers of pre-cancers, such as cervical intraepithelial neoplasia (CIN2/3) to claim effectiveness. This is a significantly lower evidentiary bar that was used to fast-track FDA approval.
The problem with this approach is well-documented. Many CIN2/3 lesions resolve naturally.
A Dutch study, for instance, tracked 114 women with CIN2/3 found that nearly two-thirds of cases regressed without intervention. Only one developed adenocarcinoma in situ (pre-cancer) and none progressed to cervical cancer.
Moreover, those lesions that don’t resolve naturally typically take years to progress, and they are usually detected through routine screening.
If CIN2/3 is an unreliable proxy for cancer, how can it serve as valid proof of Gardasil’s claimed efficacy at preventing cancer?
Are HPV Strains Merely Being Replaced?
Another major concern is “type replacement”—the possibility that suppressing certain HPV strains through vaccination leads to the rise of others.
For instance, a Finnish study found that while HPV strains 16 and 18 (targeted by the vaccine) decreased following vaccination, non-vaccine strains such as HPV 52 and 66 became more prevalent.
This raises an important question: While Gardasil may alter the landscape of HPV infections, does it actually reduce the overall risk of developing cervical cancer?
When Merck developed Gardasil 9 to target five additional HPV strains, a study involving 14,215 women found that those who received Gardasil 9 developed high-grade lesions at the same rate as those who received the original Gardasil (which only targeted four strains).

Despite the expanded coverage, the additional strains had no measurable impact on pre-cancers overall, adding to the uncertainty about whether these vaccines truly reduce cervical cancer incidence.
The Questionable Swedish and Scottish Studies
Two widely cited studies—from Sweden and Scotland—are often heralded as proof that Gardasil significantly reduces cervical cancer rates. However, Dr Lee highlights critical methodological flaws in his report.
- Swedish study
The Swedish study, published in the New England Journal of Medicine, compared cervical cancer rates between vaccinated and unvaccinated women.
However, Dr Lee points out that many participants (born between 1995 and 2007) were too young to develop cervical cancer during the study period (2006–2017).
Since cervical cancer takes decades to emerge, including these young women (ages 10–22)—who had zero cases—introduced a statistical bias that exaggerated the vaccine’s effectiveness.
Moreover, the study failed to account for the “healthy user effect,” where vaccinated individuals are more likely to engage in preventive health measures like regular screening, which independently reduces cancer risk.
As a result, attributing the decline in cancer cases solely to the vaccine is misleading.
- Scottish study
A 2024 Scottish observational study, published in the Journal of the National Cancer Institute, had similar methodological issues, and was met with sensationalist media headlines: “No cervical cancer cases in HPV-vaccinated women.”
However, Dr Lee argues this claim is deeply flawed. First, the women studied were simply too young for conclusions about long-term vaccine efficacy to be drawn.
Second, Scotland’s screening programme, which detects and treats precancerous lesions before they develop into cancer, changed its entry age in 2016 during the study period.
The age at which women were first invited for screening was raised from 20 to 25, meaning there was a 5-year gap in screening for younger women. As most cancers in women under 30 are diagnosed through screening, this change could explain any decline in cancer rates, rather than the vaccine itself.
And third, just like the Swedish study, the “healthy user effect” further confounds the results.
Despite being frequently cited as definitive proof of Gardasil’s effectiveness, these studies contain serious limitations that undermine their conclusions.
Cervical cancer screening saves lives
In developed nations, around 93% of initial HPV infections resolve without medical intervention. Cervical cancer is slow to develop, with an average onset age of 54, making long-term data essential for assessing Gardasil’s true impact.
What remains incontrovertible is the lifesaving role of cervical cancer screening.
Since the widespread adoption of Pap smears in the 1950s, cervical cancer incidence in the U.S. has plummeted—from 44 per 100,000 women in 1947 to just 8.8 per 100,000 by 1970.
This dramatic decline predates the introduction of HPV vaccination in 2006.
In Australia, deaths from cervical cancer fell significantly along with incidence following the introduction of the National Cervical Screening Programme, and remained steady despite mass HPV vaccination.
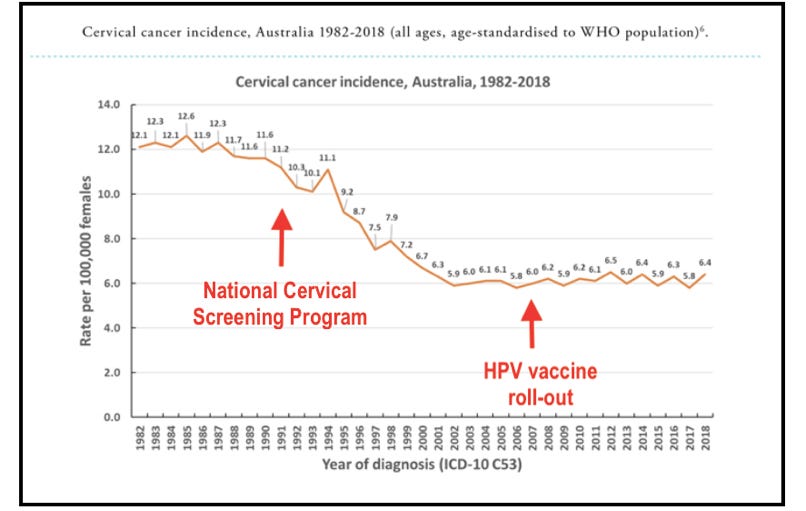
Source: https://www.hpvworld.com/articles/prevention-of-cervical-precancer-and-cancer/
Dr Nancy C. Lee, former Associate Director for Science at the CDC, testified before the U.S. Congress in 1999:
- “Cervical cancer is nearly 100 percent preventable.”
- “The most important risk factor for developing cervical cancer… is the failure to receive regular screening with a Pap smear.”
- “For a woman with CIN, her likelihood of survival is almost 100 percent with timely and appropriate treatment.”

Dr Nancy C. Lee, former Associate Director for Science at the CDC
Unlike cervical cancer, which is preventable through screening and treatable with early intervention, Dr Lee asserts the harms linked to Gardasil – such as autoimmune disorders and neurological complications – are unpredictable, difficult to treat, and often irreversible.
Did Merck Misrepresent Its Vaccine?
At the core of this legal battle is a critical question: Did Merck mislead the public about Gardasil’s true value?
Despite its widespread use, Gardasil’s long-term efficacy remains unproven, while growing evidence links the vaccine to serious harms, including autoimmune disorders and neurological complications.
For decades, cervical cancer rates have declined due to improved screening—not mass vaccination. Yet Merck has aggressively marketed Gardasil as essential for cancer prevention, even in countries where cervical cancer is already rare.
Dr Lee’s report suggests Merck selectively presented data to manufacture a false sense of necessity—one that collapses under scrutiny.
As the trial resumes in September, one question remains: Did Merck knowingly misrepresent Gardasil’s safety and efficacy, prioritising profit over public health?
“Seek them out and destroy them where they live”
Remembering Merck’s Australian doctor hit list
By John Leake | Courageous Discourse | February 3, 2025
This evening I pondered the news of Caroline Kennedy’s hit letter against her cousin, RFK, Jr., and the fact that she was the Biden Administration’s Ambassador to Australia, and the fact that she has served as a powerful ambassador for Merck’s Gardasil vaccine.
The association of Australia and Merck reminded me of the company’s “seek out and destroy” campaign against Australian doctors who expressed concern that the company’s blockbuster Vioxx seemed to be causing heart attacks and strokes. As was reported by CBS in May 2009:
Merck made a “hit list” of doctors who criticized Vioxx, according to testimony in a Vioxx class action case in Australia. The list, emailed between Merck employees, contained doctors’ names with the labels “neutralise,” “neutralised” or “discredit” next to them.
According to The Australian, Merck emails from 1999 showed company execs complaining about doctors who disliked using Vioxx. One email said:
“We may need to seek them out and destroy them where they live …”
During this same period in the United States, Merck was accused of concealing negative results of clinical Vioxx trials from the FDA and paying reputable doctors to put their names on research they did not conduct or write up. The company also published a fake journal, paying Elsevier to create a phony publication to serve as a marketing tool titled the Australasian Journal of Bone and Joint Medicine.
Ultimately the company was found guilty of knowingly concealing data about the elevated risk of stroke and heart attack from Vioxx and agreed to pay a class action settlement to stroke and heart attack victims totaling $4.85 billion.
I wonder if the nice folks at Merck would ever yield to the temptation to overstate the benefits of the HPV vaccine and downplay its risks, as some plaintiffs have alleged. I also wonder if the company’s PR department might yield to the temptation to smear RFK, Jr. during his Senate confirmation process.
Or am I just being cynical?
‘So Many Pitfalls’: Feds Push School-Based Health Centers as Critics Sound Alarm Over Lack of Parental Consent
By Suzanne Burdick, Ph.D. | The Defender | August 9, 2023
The recent push by the U.S. federal government to rapidly expand the use of school-based health centers (SBHCs) across the country has some critics concerned children will receive, or be pressured into receiving, unnecessary or unwanted medical interventions — including vaccines — without their parents’ knowledge or consent.
Georgia attorney Nicole Johnson, co-director of Georgia Coalition for Vaccine Choice and a consultant to the Children’s Health Defense’s (CHD) legal team, told The Defender :
“It’s scary because these health centers sound really good. In some of the rural and poor communities especially, this is going to seem like a really good way for children to get this care.
“And while there may be some conveniences, there are so many concerns with allowing medical exams and treatments at school. Parents need to be involved in all medical decisions and I fear they are being left out of the equation.”
SBHCs are intended to provide high-quality healthcare to kids by offering “primary care, mental health care, and other health services in schools,” particularly in underserved communities.
This includes services “to prevent disease, disability, and other health conditions or their progression” such as “immunizations” and “well-child care.”
According to the Centers for Disease Control and Prevention’s (CDC) Community Preventive Services Task Force, SBHCs can improve educational and health outcomes.
The CDC also considers SBHCs as integral to its Whole School, Whole Community, Whole Child model because they provide health services and mental health counseling.
But critics like Johnson worry that though there may be benefits to SBHCs, there are also downsides — including lack of regulation of the centers and the fact that parents may not be aware of the broad range of medical and behavioral services being provided in their children’s schools.
SBHCs have been linked to higher human papillomavirus (HPV) vaccination rates, according to a 2022 report by Harvard University’s Center for Health Law and Policy Innovation and the University of California Davis Comprehensive Cancer Center.
The report — written expressly to “address vaccine hesitancy” — concluded: “These results suggest SBHCs create a considerable opportunity … to implement successful school based HPV vaccination programs.”
Merck, the maker of the Gardasil HPV vaccine, is one of the funders of the School-Based Health Alliance, a large networking organization that “works on policy, standards, data, and training issues” regarding SBHCs.
Federal, state authorities pour taxpayer money into school-based health centers
The idea of running full-service health centers in public schools has been around for more than two decades, but events in 2022 caused SBHCs to catch on like wildfire.
Congress and President Joe Biden in June 2022 passed the Bipartisan Safer Communities Act, which allowed the U.S. Department of Health and Human Services (HHS) to award $50 million in grants to states “for the purpose of implementing, enhancing, or expanding the provision” of healthcare assistance through SBHCs using Medicaid or the Children’s Health Insurance Program (CHIP).
The legislation charged the Centers for Medicare & Medicaid Services (CMS) with expanding access to Medicaid healthcare services — including behavioral health services — in schools, and reducing the administrative burden for states and schools.
A CMS spokesperson told The Defender that Medicaid and CHIP now can provide reimbursement for services given in SBHCs for children and youth who are covered by those programs.
Additionally, in May 2022 HHS awarded $25 million in grants to 125 SBHCs “to improve and strengthen access to school-based health services in communities across the country.”
State public officials also are dedicating funds to expand SBHCs. For instance, the governor of Georgia in fall 2022 announced an investment of $125 million to expand school-based health services to rural communities in Georgia.
Pediatricians can ‘partner’ with schools
The American Academy of Pediatrics (AAP) supports SBHCs and said in a policy statement that pediatricians may act as “sponsors” by partnering with a school to establish the SBHC as an extension of their practice or by supervising the care given at a SBHC.
“Sponsors also include local hospitals that can provide prearranged after-hours and school vacation coverage and financial support for SBHCs,” the AAP said.
The Defender reached out to the AAP statement’s lead authors for comments on how parental consent is handled in SBHCs, but they did not respond by our publication deadline.
SBHCs also have the support of the School-Based Health Alliance. In addition to funding from Merck, the alliance receives financial support from HHS’ Health Resources & Services Administration.
Documents obtained in June by CHD revealed that the HHS gave $4.7 million to research headed by a Merck consultant that focused on developing “The Announcement Approach Training,” where providers simply “announce” a child will be receiving the HPV vaccine as part of a routine office visit, instead of discussing it with the family first.
The government-funded research also is testing whether financial incentives and peer pressure can “nudge” doctors to change how they talk to their patients in order to increase HPV vaccine uptake among adolescents.
Meanwhile, a fierce battle is taking place in multiple states where some lawmakers are pushing legislation that would allow minors to receive treatments to prevent sexually transmitted diseases — including Merck’s HPV vaccine — without parental knowledge or consent.
‘So many pitfalls … so many ways for someone else to be making parental decisions’
Justine Tanguay, an attorney with nearly 20 years of experience advocating for children in various areas of the law, told The Defender :
“Don’t be fooled! This year many schools will be sending home blanket consent-to-treat forms for parents to sign.
“Parents need to be aware that these forms are not the traditional authorization requests for the school nurse to give first-aid or to treat minor illnesses.”
Tanguay, CHD’s director of campaign and research, explained that the forms may give those who run the SBHC the legal authorization to provide “comprehensive healthcare.”
This could include — but may not be limited to — “the ability to provide preventative treatment, behavioral and mental health services, reproductive counseling, lab and prescription services, various medical screenings, immunizations and disease management,” Tanguay said.
Moreover, SBHC staff will have “direct access” to a minor child, Tanguay said, “as well as the ability to encourage a minor child to make personal healthcare decisions without the need to consult with and seek approval from a parent.”
“The opportunity to circumvent both parental rights and informed consent is ripe for abuse,” Tanguay warned.
Johnson agreed, saying, “There are just so many pitfalls here, so many ways for someone else to be making parental decisions.”
Johnson shared with The Defender a consent form currently used in a school district north of Atlanta, Georgia.
The form says nothing about parents being notified before, during or after treatment. It reads:
“I hereby voluntarily give my consent for [my child] to receive health services with Georgia Highlands Medical Services at Cumming Elementary School.
“I further authorize any health care provider and professional staff working for the clinic to provide such medical tests, diagnoses, procedures, and treatments as are reasonably necessary or advisable for the medical evaluation and management of my child’s health care.”
The form does not clarify who determines what services are “reasonably necessary or advisable” and does not explain how parents will be involved in that process. It states:
“I understand that my signing this consent allows the health care provider and professional clinic staff of Georgia Highlands Medical Services at Cummings Elementary Schools to provide comprehensive health services which includes physical and behavioral health services.”
Again, the form does not clarify what specifically falls into the category of “physical and behavioral health services” or how parents will be involved in the determination for what services their child may need.
“I think about my own kids when they were in school,” Johnson said, “how easily they could have been swayed to get a vaccine or a medical treatment just because an adult told them that they should.”
“It’s really dangerous to have all of these things offered to them without the parents even being aware,” she said. “A lot of kids — most kids — are compliant. They want to do what the adults are telling them to do.”
According to the CDC, a key component of its Whole School, Whole Community, Whole Child model, which includes SBHCs, is “family engagement.”
However, the agency’s 37-page document about family engagement mentions parental permission only once and does not discuss parental consent for medical treatment beyond the application of sunscreen during recess.
According to a CMS spokesperson, SBHCs “follow the same practices as any other medical center or Medicaid or Children’s Health Insurance Program (CHIP) provider … including parental consent requirements.”
The spokesperson did not go into detail on whether consent would be requested generally or for each specific medical treatment.
Where’s the regulatory oversight?
Tanguay pointed out that SBHCs exist without proper regulatory oversight.
According to Stand for Health Freedom, a nonprofit “dedicated to protecting informed consent in medical care,” SBHCs are “completely unregulated.”
For instance, it is presently unclear how HIPAA law (the Health Insurance Portability and Accountability Act of 1996) and FERPA law (the Family Educational Rights and Privacy Act) will be applied to SBHCs and students’ health information.
Stand for Health Freedom also pointed out that although in-school clinics may relieve busy parents of the burden of taking their children to the doctor, “medical ethics do not allow physicians to treat minors without a parent or guardian present, which is why parents cannot simply drop their child off at the doctor’s office and come back later to collect them.”
Stand for Health Freedom said:
“Parents must engage politically and work with state health freedom leaders to ask lawmakers to either ban SBHCs in favor of the existing limited school-nurse model, or place guardrails on SBHCs to protect parental consent and involvement in their minor children’s medical care.”
Meanwhile, proponents of SBHCs, such as the School-Based Health Alliance, argue that SBHCs are a “powerful tool for achieving health equity among children and adolescents who unjustly experience disparities in outcomes simply because of their race, ethnicity, or family income.”
Johnson said she disliked being “so skeptical of something that may potentially benefit some people” but added, “as a parent, it is your job and your right to be a part of the decisions that affect the health and well-being of your child.”
Johnson said parents who experienced or witnessed vaccine injury would be particularly skeptical of putting medical decisions in the hands of government agencies, including schools.
“And the COVID response created even more skeptics,” she said, adding:
“It’s unfortunate that we have to approach this [SBHCs] with the thought, ‘How could this be abused?’ But that’s where we are.”
The Defender on Aug. 3 reached out to the School-Based Health Alliance to ask how parental consent in SBHCs is handled and what they’d like parents who may feel distrustful of the U.S. medical system to know about SBHCs. The alliance did not respond by our publication deadline.
Suzanne Burdick, Ph.D., is a reporter and researcher for The Defender based in Fairfield, Iowa. She holds a Ph.D. in Communication Studies from the University of Texas at Austin (2021), and a master’s degree in communication and leadership from Gonzaga University (2015). Her scholarship has been published in Health Communication. She has taught at various academic institutions in the United States and is fluent in Spanish.
This article was originally published by The Defender — Children’s Health Defense’s News & Views Website under Creative Commons license CC BY-NC-ND 4.0. Please consider subscribing to The Defender or donating to Children’s Health Defense.
Utah Woman Is First to Sue Merck Alleging Gardasil HPV Vaccine Caused Cervical Cancer
The Defender | April 26, 2023
A lawsuit filed Tuesday in federal court alleges Merck’s Gardasil human papillomavirus (HPV) vaccine caused a young Utah woman to develop cervical cancer and other injuries.
The lawsuit, filed on behalf of Caroline Cantera, 25, by Wisner Baum (formerly Baum Hedlund Aristei & Goldman), is the first lawsuit to allege Gardasil can cause cervical cancer — the very cancer Merck asserts Gardasil prevents.
Cantera alleges New Jersey-based Merck & Co., Inc., and subsidiary Merck Sharp & Dohme oversold Gardasil as a “cervical cancer vaccine” and downplayed known health risks to enhance sales.
Cantera’s attorneys filed the complaint in the U.S. District Court for the Western District of North Carolina as part of the Gardasil multidistrict litigation (MDL). Dozens of federal Gardasil injury cases filed throughout the country have been consolidated in North Carolina.
According to the complaint, Merck never studied whether Gardasil prevents cervical cancer. Instead, the drugmaker tested Gardasil to determine if it could prevent the development of certain lesions, some of which are considered related to cancer — even though a majority of such lesions, even the most serious, regress on their own.
Public health officials have long recommended the Pap test as the most effective frontline public health response to prevent cervical cancer. Long before Gardasil was introduced to the market in 2006, cervical cancer rates had been plummeting by up to 80% with the implementation of routine Pap testing.
For those who are diagnosed with precancerous lesions or worse, cervical cancer is largely treatable if caught early.
Nonetheless, Merck sought “Fast Track” U.S. Food and Drug Administration approval of Gardasil. Once approved, Merck engaged in a relentless marketing campaign falsely proclaiming that Gardasil was a “cervical cancer vaccine” and that any young girl vaccinated with Gardasil would become “one less” woman with cervical cancer, the lawsuit claims.
Cantera alleges Gardasil can actually increase the risk of cervical cancer. The Gardasil vaccine label specifically states, “Gardasil has not been evaluated for potential to cause carcinogenicity or genotoxicity.”
Studies from the Centers for Disease Control and Prevention (CDC) suggest the suppression of the HPV strains targeted by Gardasil (there are more than 200 HPV strains and Gardasil targets between four and nine strains) may actually open an ecological niche for replacement by more virulent strains, thus increasing the risk of cervical cancer.
Merck’s own studies show that for those previously exposed to HPV (a huge percentage of the population) when vaccinated, there is an up to 44.6% increased risk of developing advanced abnormal pre-cancer cells or worse.
The complaint also cites rapidly climbing cervical cancer rates among young women in countries where Gardasil has seen a high uptake. Studies also show young women who received the Gardasil vaccine are foregoing routine Pap screening due to a false sense of security that the HPV vaccine will protect them from cervical cancer.
Bijan Esfandiari, co-lead counsel for plaintiffs in the Gardasil MDL, said:
“Merck has heavily promoted Gardasil as a cancer prevention vaccine even though the studies weren’t designed to answer that question, and its marketing has effectively resulted in young women foregoing Pap screening, the most reliable and proven method of preventing cervical cancer.
“Merck also hasn’t studied whether Gardasil can cause cancer, but we now have evidence it can increase the risk of cancer. Given Merck makes over $6 billion a year on Gardasil, it has little incentive to stop the deception.
“Through our litigation, we hope to expose the truth and hold Merck accountable for the harm it has done to Caroline and others.”
Cantera ‘had to face the painful fact that I will never be able to have children of my own’
Cantera was 19 years old when she received her first of three Gardasil shots. She said she agreed to receive Gardasil after being convinced by Merck’s prolific marketing that the vaccine is very safe and prevents cervical cancer.
Before receiving the HPV vaccine, Cantera was healthy and never had to go to the doctor for anything other than regular checkups and physical exams for sports. She received routine Pap tests, all of which were negative prior to Gardasil.
In high school, she played tennis, regularly went backpacking and loved spending time outdoors. She lived a happy, carefree life filled with friends and activities.
After her Gardasil injections, Cantera experienced unexpected fatigue, intense stomach aches and overall weakness throughout her body. The fatigue and occasional abdominal pain continued until she noticed that her period had lasted over four weeks.
After what she thought was an initial visit to a gynecologist, her life suddenly took a drastic and unexpected turn.
Cantera was diagnosed with stage four cervical cancer. She received multiple biopsies, CT scans and MRIs, had six rounds of chemotherapy, 30 radiation treatments, three brachytherapy treatments and saw countless doctors.
She was unable to go back to university for her final semester and struggled to finish the classwork necessary to receive her undergraduate degree.
Because most of her treatment was directed at her cervix, her ovaries were also affected, putting her into menopause in her 20s. She will never be able to have children of her own because her eggs are no longer viable due to the cancer treatment.
Cantera said:
“Every day since my diagnosis has been a battle. My body is still recovering from the toll of such intense treatments to fight cancer, and I live in constant fear that my cancer could come back at any time.
“On top of all that, I also had to face the painful fact that I will never be able to have children of my own. If Merck knew this vaccine can cause so much harm, why didn’t they warn people?”
In September 2022, Wisner Baum and Robert F. Kennedy Jr., chairman on leave from Children’s Health Defense, filed their first wrongful death suit against Merck, alleging the drugmaker’s Gardasil HPV vaccine caused the death of 13-year-old Noah Tate Foley.
Wisner Baum and Kennedy have filed numerous lawsuits against Merck alleging the company knowingly conceals the adverse events associated with its Gardasil vaccine. These include:
- Victoria Trevisan (California)
- Merrick Brunker (California)
- Emma Sullivan (New Jersey)
- Ashley Dalton (Michigan)
- Abigail Stratton (South Carolina)
- Savannah Flores (Nevada)
- Korrine Herlth (Connecticut)
- Kayla Carrillo (California)
- Michael Colbath (California)
- Sahara Walker (Wisconsin)
- Zachariah Otto (California)
- Julia Balasco (Rhode Island)
While each case is unique, all of the plaintiffs agree that if Merck had told the truth about the known dangers associated with Gardasil, they never would have consented to the HPV vaccine.
If you or your child suffered harm after receiving the Gardasil HPV vaccine, you may have a legal claim. Visit Wisner Baum for a free case evaluation or call 855-948-5098.
This article was originally published by The Defender — Children’s Health Defense’s News & Views Website under Creative Commons license CC BY-NC-ND 4.0. Please consider subscribing to The Defender or donating to Children’s Health Defense.
Merck’s Taxpayer-Subsidized COVID Pill Linked to New Virus Mutations, Study Finds
By Michael Nevradakis, Ph.D. | The Defender | February 3, 2023
Merck’s oral antiviral pill for COVID-19, molnupiravir — marketed under the name Lagevrio — may be fueling the development of new and potentially deadly variants of COVID-19, according to the authors of a new preprint study.
The study, released Jan. 27 by a team of U.S. and U.K researchers, found, “It is possible that some patients treated with molnupiravir might not fully clear SARS-CoV-2 infections, with the potential for onward transmission of molnupiravir-mutated viruses.”
Merck received significant taxpayer funding from the Biden administration to develop and distribute molnupiravir, and the U.S. government bought nearly 2 million courses of the drug on the taxpayer’s dime.
The study, which is pending peer review, followed the discovery by a middle school science and math teacher in Indiana who found numerous variants of COVID-19 emerged after molnupiravir began to be widely distributed.
Scientists had long warned that the development of such mutations from the use of molnupiravir was possible.
“It’s not a surprise that molnupiravir could cause [the] escape of mutant virus strains or substrains into the population,” said Dr. Harvey Risch. “Its main function is to get the virus to mutate faster.”
Risch, professor emeritus and senior research scientist in epidemiology (chronic diseases) at the Yale School of Public Health, told The Defender :
“The idea is that it will mutate itself to death. But some live mutants could get out, and this paper gives evidence that they have.”
Brian Hooker, Ph.D., P.E., chief scientific officer for Children’s Health Defense, said the study’s authors scanned global SARS-CoV-2 sequence databases looking for mutations characteristic of those by molnupiravir (G-to-A and C-to-U) and found an uptick of those mutants starting in 2022 — after molnupiravir was put on the market and specifically in countries where molnupiravir was distributed.
“Although this isn’t ‘direct proof’ that the mutations came directly from molnupiravir use,” Hooker told The Defender, “the evidence is very compelling, confirming the fears of many who warned of this prior to FDA [U.S. Food and Drug Administration] approval of the drug in late 2021.”
The FDA granted molnupiravir Emergency Use Authorization (EUA) on Dec. 23, 2021, for use in mild-to-moderate COVID-19 infections in patients 18 and over.
The EUA came just one day after the FDA authorized Pfizer’s COVID-19 antiviral treatment Paxlovid.
Merck this week announced massive revenues from sales of molnupiravir in 2022, but projected a significant decrease in those sales in 2023.
The FDA on Wednesday removed the requirement that a person has to test positive for COVID-19 in order to get a prescription for molnupiravir or Paxlovid.
‘I think we are courting disaster’
Molnupiravir “works by creating mutations in the COVID-19 genome that prevent the virus from replicating in the body, reducing the chances it will cause severe illness,” according to Bloomberg.
However, according to Science, the findings of the preprint study suggest “some people treated with the drug generate novel viruses that not only remain viable, but spread.”
This finding “underscores the risk of trying to intentionally alter the pathogen’s genetic code,” leading some researchers to “worry the drug may create more contagious or health-threatening variations of COVID,” Bloomberg reported.
Virologist William Haseltine, Ph.D., chair and president of ACCESS Health International, has repeatedly raised such concerns about molnupiravir.
“It’s very clear that viable mutant viruses can survive [molnupiravir treatment] and compete [with existing variants],” Haseltine told Science. “I think we are courting disaster.”
According to the Gateway Pundit, “When one studies how Lagevrio works, this should not come as a shock. The pill attacks the COVID virus by trying to alter its genetic code.”
The Gateway Pundit reported:
“Once inside a human cell, a virus can make 10,000 copies of its genetic code in a few hours. Each copy made increases the risk the virus makes a rare mistake and creates an inexact replica.
“This is how mutations happen as we have seen with COVID. A drug that deliberately alters a virus’s genetic code would greatly increase the mutation risk.”
Dr. Jonathan Li, a virologist and the director of Li Laboratory, associated with Harvard Medical School and Brigham and Women’s Hospital, told Bloomberg :
“There’s always been this underlying concern that it could contribute to a problem generating new variants. This has largely been hypothetical, but this preprint validates a lot of those concerns.”
According to Science, Haseltine and other scientists have long worried that molnupiravir would create COVID-19 mutations that “would survive and propagate — and perhaps turn out to be more transmissible or virulent than before.”
A Merck spokesperson described that theory as “an interesting hypothetical concern,” prior to the drug receiving EUA.
The same scientists also worried that aside from the virus, the DNA of those receiving the drug might also mutate, Science reported.
These concerns led “researchers and citizen scientists” to examine COVID-19 genome sequences cataloged in the international GISAID (Global Initiative on Sharing Avian Influenza Data) database, seeking to identify mutations likely to be caused by molnupiravir.
‘Clearly something is happening here’
Searching for these mutations was based on the premise that, “Rather than inducing random changes in the virus’ RNA genome, [molnupiravir] is more likely to cause specific nucleic acid substitutions, with guanine switching to adenine and cytosine to uracil,” added Science.
Through this process, Ryan Hisner, a middle school science and math teacher from Monroe, Indiana — described by Science as a “virus hunter” — ultimately “identified dozens of sequences that showed clusters of those hallmark substitutions.”
Hisner took to Twitter with his concerns, where he came into contact with Thomas Peacock, Ph.D., a virologist at the Imperial College London. They and other U.K. and U.S. researchers “systematically reviewed more than 13 million SARS-CoV-2 sequences in GISAID and analyzed those with clusters of more than 20 mutations,” according to Science.
The team found “a large subset showed the hallmark substitutions; all dated from 2022, after molnupiravir began to be widely used,” Science reported.
According to the preprint study, Molnupiravir, “acts by inducing mutations in the virus genome during replication. Most random mutations are likely to be deleterious to the virus, and many will be lethal.”
However, the researchers wrote:
“It is possible that some patients treated with molnupiravir might not fully clear SARS-CoV-2 infections, with the potential for onward transmission of molnupiravir-mutated viruses.
“We set out to systematically investigate global sequencing databases for a signature of molnupiravir mutagenesis. We find that a specific class of long phylogenetic branches appear almost exclusively in sequences from 2022, after the introduction of molnupiravir treatment, and in countries and age groups with widespread usage of the drug.
“Our data suggest a signature of molnupiravir mutagenesis can be seen in global sequencing databases, in some cases with onwards transmission.”
Peacock told Science these “signature clusters” were up to 100 times more likely to be identified in countries where molnupiravir was widely used, including the U.S., U.K. and Australia, as compared to countries such as Canada and France, where it was not in widespread use.
“Clearly something is happening here,” said Peacock.
Merck: ‘no evidence’ any antiviral agent has contributed to the emergence of circulating variants’
Theo Sanderson, Ph.D., a geneticist at the Francis Crick Institute and co-author of the preprint, told Science “We are not coming to a conclusion about risk” just yet, with regard to whether or not these mutations may lead to more severe COVID-19 variants.
Indeed, according to the preprint study, the variants identified by the researchers have not been shown to be more lethal or more evasive to immunity than other existing strains of COVID-19.
However, Haseltine illustrated the potential risk via the analogy of owning a pet lion: “Just because it didn’t bite you yesterday doesn’t mean it won’t bite you today.”
According to the Gateway Pundit :
“Merck was warned by multiple scientists their drug might create problematic mutations which would render the virus more dangerous and difficult to treat. The company decided to blow off any concerns and put Lagevrio [molnupiravir] on the market anyway.”
As previously reported by The Defender, Dr. James Hildreth, president and CEO of Meharry Medical College and member of Biden’s COVID-19 Health Equity Task Force, expressed concerns about mutant variants escaping.
In 2021, Hildreth told an FDA advisory panel, “Even if the probability is very low, one in 10,000 or 100,000, that this drug would induce an escape mutant from which the vaccines we have do not cover, that could be catastrophic for the whole world actually.”
Also in 2021, Haseltine told Science :
“You are putting a drug into circulation that is a potent mutagen at a time when we are deeply concerned about new variants. I can’t imagine doing anything more dangerous.
“If I were trying to create a new and more dangerous virus in humans, I would feed a subclinical dose [of molnupiravir] to people infected.”
Two other recent studies also called out molnupiravir, questioning its effectiveness and raising concerns the drug may help lead to the development of new COVID-19 variants.
A December 2022 preprint by a team of Australian researchers, found “this commonly used antiviral can ‘supercharge’ viral evolution in immunocompromised patients, potentially generating new variants and prolonging the pandemic.”
And a study published Jan. 28 in The Lancet found, “Molnupiravir did not reduce the frequency of COVID-19-associated hospitalisations or death among high-risk vaccinated adults in the community.”
University of Cambridge clinical microbiologist Ravindra Gupta, Ph.D., told Science that while it’s unclear whether molnupiravir will cause deadlier COVID-19 variants, the overall results of these recent studies “call into question whether molnupiravir should be used.”
Merck spokesperson Robert Josephson defended the product, telling Bloomberg, “There is no evidence that any antiviral agent has contributed to the emergence of circulating variants.”
Molnupiravir ‘different’ than Paxlovid — and ‘riskier’
Although molnupiravir is similar to Paxlovid in that both are oral antiviral treatments for COVID-19, Hooker told The Defender there are significant differences in how the two drugs work:
“Molnupiravir acts on the SARS-CoV-2 virus by directly inducing mutations in the RNA genome. This is a completely different mode of action compared to Pfizer’s product, Paxlovid, and in my estimation is quite dangerous.
“Merck claimed the mutation rate induced by molnupiravir would kill the virus and that mutants wouldn’t escape, but that has been shown to be false in studies of immunocompromised patients.”
Hooker said Paxlovid — and the COVID-19 vaccines — can potentially lead to the development of mutations as well.
But in his view, the “mechanism of action” used by molnupiravir is different — and far riskier — than Paxlovid and COVID-19 vaccines, which merely increase the virus’ lifetime in the human body, giving the virus a greater opportunity to naturally mutate.
Hooker said:
“In contrast, molnupiravir directly induces mutations and thereby vastly increases the mutation rate of the virus in the human host.
“In my estimation, this is a very dangerous way to treat such an infection, given the implications of creating random mutants.”
Merck made billions from molnupiravir — thanks to taxpayers
In 2022, sales of Merck’s molnupiravir hit $5.68 billion, fueled in part by strong fourth-quarter sales of the drug in Asia.
Fourth-quarter sales of molnupiravir reached $825 million, more than doubling analyst expectations of $358 million.
These strong earnings were boosted by government — or taxpayer — support.
In June 2021 — with molnupiravir still in clinical trials, which weren’t completed until October 2021 — the federal government signed a $1.2 billion contract with Merck for 1.7 million courses of the drug, at a cost of approximately $712 per patient.
An analysis by Melissa Barber of the Harvard T.H. Chan School of Public Health and Dzintars Gotham of King’s College Hospital in London found the cost of production of molnupiravir was approximately $1.74 per unit — or $17.74 for a five-day regimen.
By those calculations, the U.S. government paid a near-4,000% markup.
In March 2022, during his State of the Union address, President Biden announced the “Test to Treat” initiative, which allowed those who tested positive for COVID-19 at a pharmacy to obtain free antiviral pills — including molnupiravir — on the spot.
One month earlier, the Biden administration had proceeded with a new purchase of 3.1 million courses of molnupiravir, with the option to purchase more.
Estimates for Merck, and other COVID-19 drugmakers, are less rosy for 2023, as the public tires of all things pandemic and Biden looks to end the COVID-19 national emergency in May.
According to Reuters, sales of molnupiravir are expected to fall to about $1 billion this year, contributing to an expected decline in sales for Merck from $59.3 billion in 2022 to $57.2-$58.7 billion this year.
Merck’s stock price dropped by about 2% with Thursday’s announcement.
Despite these large earnings, overall sales of molnupiravir lagged significantly behind Paxlovid in 2022. Sales of Paxlovid reached $18.9 billion last year.
Michael Nevradakis, Ph.D., based in Athens, Greece, is a senior reporter for The Defender and part of the rotation of hosts for CHD.TV’s “Good Morning CHD.”
This article was originally published by The Defender — Children’s Health Defense’s News & Views Website under Creative Commons license CC BY-NC-ND 4.0. Please consider subscribing to The Defender or donating to Children’s Health Defense.
WHO Wants To Run the World?
By Paul Frijters, Gigi Foster, Michael Baker | Brownstone Institute | July 11, 2022
In Geneva in late May at the 75th meeting of the WHO’s decision-making body, the World Health Assembly (WHA), amendments to its International Health Regulations (IHRs) were debated and voted upon. If passed, they would grant the WHO the right to exert unconscionable pressure on countries to accept the WHO’s authority and health policy actions if the WHO decides that there is a public health threat that might spread beyond a country’s borders.
As Ramesh Thakur, the second man at the UN for years, noted, the amendments would mean “the rise of an international bureaucracy whose defining purpose, existence, powers and budgets will depend on outbreaks of pandemics, the more the better.”
This is the first clear instance of a globalist coup attempt. It would subvert national sovereignty worldwide by putting real power into the hands of an international group of bureaucrats. It has long been suspected that the authoritarian elites arisen during covid times would try to strengthen their positions by undermining nation states, and the this 75th jamboree is the first solid evidence of this being true.
What an opportunity then to see who is in the conspiring club. Who drafted the amendments? What was in them? Which individuals supported them or spoke out against them?
WHO were the conspirators?
The amendments on the table at the May WHA meeting had been transmitted to the WHO by the US Department of Health and Human Services on January 18, circulated by WHO to its member states (‘States Parties’) on January 20 and formally introduced to the WHA on April 12.
The proposals, according to an announcement on January 26, were co-sponsored by 19 countries plus the European Union. Even if some co-sponsors had little direct involvement in drafting them, they all would have approved in principle the overarching goal of tightening up the WHO’s authority over member states in the face of a public health event.
Loyce Pace, the HHS’s Assistant Secretary for Global Affairs – the leading US official nominally responsible for the proposed amendments – arrived at the Biden administration fresh from a stint as executive director of an advocacy organization called the Global Health Council.
That council receives funding from the Bill & Melinda Gates Foundation and its members include Eli Lilly, Merck, Pfizer, Abbott Labs, and Johnson & Johnson. You get the idea. Via one of the foxes-turned-chicken-guard, it appears the HHS ‘worked closely’ on these amendments with large pharmaceutical companies, who will be chomping at the bit for a more proactive (read: profitable) response to any public health emergency, real or imagined.
So the conspiring club consists primarily of the US government and its Western allies in lockstep with Big Pharma, and they are looking to undermine both the sovereignty of their own governments and that of other countries, presumably with the idea that the Western elites would do the running.
What was in them? A blizzard of acronyms and euphemisms
To understand what the US proposed at the WHA, we need first to understand how things have worked in the WHO to this point.
The IHRs in their current form have been in force as international law since June 2007. Among other things, they impose requirements on countries to detect, report and respond to ‘public health events of international concern,’ or PHEICs. The WHO Director-General consults with the state where a possible public health event has occurred, and within 48 hours they are meant to come to a mutual agreement on whether or not it actually is a PHEIC, whether or not it needs to be announced to the world as such, and what counter-measures, if any, should be taken. It’s essentially an early-warning system on major health crises. This is a good thing if it’s run by people you can trust and if it has checks and balances to rein in expansionary tendencies.
The proposed amendments would greatly strengthen the power of the WHO relative to this baseline, in a number of ways.
First, they lower the threshold for the WHO to declare a public health emergency by empowering its Regional Directors to declare a ‘public health event of regional concern’ (PHERC, italics ours) and for the WHO to put out a new thing called an ‘intermediate public health alert.’
Second, they permit the WHO to consider allegations about a public health event from non-official sources, meaning sources other than the government of the state concerned, and allow that government only 24 hours to confirm the allegations and a further 24 hours to accept the WHO’s offer of ‘collaboration.’
Collaboration is essentially a euphemism for on-site assessment by teams of WHO investigators, and concomitant pressure at the whim of WHO personnel to enact potentially far-reaching measures such as lockdowns, movement restrictions, school closures, consumption of medicines, administration of vaccines and any or all of the other social, economic, and health paraphernalia that we have come to associate with the covid circus.
Should the state’s government acceptance of the WHO’s ‘offer’ not be forthcoming, the WHO is empowered to disclose the information it has to the other 194 WHO countries, while continuing to pressure the state to yield to the WHO’s invitation to ‘collaborate.’ A non-collaborating country would risk becoming a pariah.
Third, the proposal includes a new Chapter IV, which would establish a ‘Compliance Committee’ consisting of six government-appointed experts from each WHO region tasked with permanently nosing around to ensure the member states are complying with IHR regulations.
There are more crossings-out of the existing IHR language and new language added in, but the flavour of what the US-led alliance is shooting for is a WHO that can unilaterally decide whether there is a problem and what to do about it, and can isolate countries that disagree.
Compliant WHO member states could act as a supporting cast in the isolation effort, through the distribution of their own health budgets and their ‘health-related’ policies, which would include travel and trade restrictions. The WHO would become a kind of command-and-control center for globalist agendas, pushing the produce of (Western) Big Pharma.
Why and how would this work?
We learned during covid times why it would make sense that the US and its allies are insisting on these amendments.
Lowering the bar for declaring a global (or regional) public health threat triggers a huge opportunity for Western pharmaceutical companies. As legal experts have observed: “WHO emergency declarations can trigger the fast-track development and subsequent global distribution and administration of unlicensed investigational diagnostics, therapeutics and vaccines.
This is done via the WHO’s Emergency Use Listing Procedure (EULP). The introduction of an ‘intermediate public health alert’ in particular will also further incentivise the pharmaceutical industry’s move to activate domestic fast-track emergency trial protocols as well as for advance purchase, production and stockpile agreements with governments before the existence of a concrete health threat to the world’s population has been detected, as is already the case under WHO’s EULP via the procedures developed for a ‘pre-public health emergency phase’.”
You can bet that the WHO ‘expert teams’ sent in to make on-the-ground assessments, under the banner of ‘collaboration’ with the host country experiencing the health event, will be chock-a-block with operatives from the CDC and who knows what other Western agencies, all poking around potentially sensitive facilities that a host government might justifiably claim a sovereign right to keep to itself. Likewise with the ‘Compliance Committee’ proposed by the US under the new Chapter IV of the IHRs: its government-appointed members have an open-ended brief, enshrined in international law, to be busybodies.
In layman’s terms, the WHO would be turned into an international thug, with its member states offered the role of backyard gang members.
As a bonus for Western elites, the proposals are a sneaky form of rewriting history. By cementing authority within an international organisation to determine the existence of public health crises and direct potentially draconian emergency responses, Western governments would get to enshrine and legitimise their own extreme responses to the covid outbreak, as we have pointed out previously. Their backsides would thereby be given some protection from legal challenges.
The refusniks: Developing countries
The proposals were pushed primarily by Western countries: the US was joined by Australia, the UK and the EU in arguing for passage. The resistance was led by developing countries who saw it as a colonialist ambush in which their ability to set policy and respond to health threats in a manner commensurate with their domestic situations would be overridden.
Brazil reportedly went so far as to threaten to withdraw from the WHO, and the African group of almost 50 countries, along with India, argued that the amendments were being rushed through without adequate consultation. Russia, China and Iran also objected.
Failure on the first try, but the US and its allies in the West will get more shots to push it through.
How do we expect them to do this? Well, when a proposal gets bogged down inside a giant bureaucratic machine like the WHO, the inevitable response is to set up committees to work in the background and circle back with a new set of proposals to be presented at a future meeting. True to form, a ‘working group’ and ‘expert committee’ are being assembled to accept member state proposals on IHR reform by the end of September this year. These will be ‘sifted through’ and reports will be prepared for review by the WHO’s executive board in January next year. The objective is to have a fresh set of proposals on the table when the WHA convenes for the 77th time in 2024.
Not all was lost
Salvaging something from the fact that the WHA failed to get a consensus around its biggest agenda item, the US and its allies got a small victory on the point of when they can try again – though in their desperation they needed to violate the IHRs’ own rules to accomplish it. Article 55 of the IHRs states unambiguously that a four-month notice period is required for any amendments.
In this instance, revised amendments were presented on May 24, the same day that the first lot were rejected. These were discussed, further amended on May 27 and then adopted on the same day. The approved amendments halve the two-year period for any (further) approved amendments to the IHRs to take effect. (The IHRs that came into force in 2007 were agreed to in 2005 – but under the new resolution, anything agreed to in 2024 would come into effect in 2025 rather than 2026.)
Yet, what was achieved in terms of fast-tracking the force of new amendments was lost in slow-tracking their implementation. Nations would have up to 12 months – double the previous suggestion of six months – to implement any IHR amendments that newly enter into force of law.
State of play
Where is all this going?
If the WHO takes the reins on decisions about what constitutes a health crisis, and can pressure every country into a one-size-fits-all set of responses that it, the WHO, also determines, that’s bad enough. But what about if its invitation to ‘collaborate’ with countries is backed up with teeth, such as sanctions against those who demur? And what about if it then broadens the definition of ‘public health’ by, for example, declaring that climate change falls under that definition? Or racism? Or discrimination against LBTQIA+ people? The possibilities thereby opened up for running the world are endless.
A global ‘health’ empire would bring huge harms to humanity, but a lot of power and money is pushing for it. Don’t think it can’t happen.
Paul Frijters is a Professor of Wellbeing Economics at the London School of Economics: from 2016 through November 2019 at the Center for Economic Performance, thereafter at the Department of Social Policy
Pfizer Document Dump Shows Doctor With Ties to Gates Foundation Deleted Trial Participant’s Vaccine Injury
By Michael Nevradakis, Ph.D. | The Defender | May 18, 2022
An 80,000-page cache of Pfizer-BioNTech COVID-19 vaccine documents released by the U.S. Food and Drug Administration (FDA) sheds light on Pfizer’s extensive vaccine trials in Argentina, including the unusually large size of the trials and the story of a trial participant whose vaccine reaction was “disappeared.”
The case of Augusto Roux in Argentina suggests that in at least one instance, a trial participant whose symptoms were determined to be connected to the COVID-19 vaccine was later listed, in official records, as having experienced adverse events that were not related to the vaccination.
Vaccine trials in Argentina also appear to have glossed over adverse events suffered by other trial participants, and the potential connection between the adverse events and the vaccine.
The FDA on May 2 released the latest cache of documents, which pertain to the Emergency Use Authorization of Pfizer’s vaccine, as part of a court-ordered disclosure schedule stemming from an expedited Freedom of Information Act request filed in August 2021.
As previously reported by The Defender, the documents included Case Report Forms from Pfizer COVID vaccine trials in the U.S., and the “third interim report” from BioNTech’s trials conducted in Germany, both of which listed adverse events sustained by participants in the U.S. and German trials.
Many of these adverse events were indicated as being “unrelated” to the vaccines — even in instances where the patients were healthy or otherwise had no prior medical history related to the injuries they sustained.
Story of ‘disappeared patient’ goes public
Several bloggers and online investigators called into question various aspects of the Argentine vaccine trials, pointing out the number of participants in the Argentine trials dwarfed that of other, typically smaller trials at other locations in different countries.
They also pointed out the large number of participants appeared to have been recruited to the trial in a remarkably short time, and questioned the connections between one of the key figures of the Argentine trial to vaccine manufacturers, Big Pharma and the Bill & Melinda Gates Foundation.
The large number of trial participants in Argentina may be related to the fact that the trial appears to have been held simultaneously in 26 hospitals.
The large number of participants is revealed in another of the documents released this month, where on page 2,245, the list of randomized participants at trial site 1231 begins, while on page 4,329, the list of participants at trial site 4444 begins.
Site 1231 refers to the main trial site location and 4444 (page 24) most likely refers to the disparate hospitals participating in the trial outside the main location.
Commenting on the revelation, blogger David Healy wrote:
“About 5,800 volunteers were enrolled, half getting the active vaccine. This is almost 4 times more than the next largest centre in this trial.
“Amazingly 467 doctors were almost instantly signed up and trained as assistant investigators in the study.”
In all, 4,501 patients participated in the Argentine trials, representing 10% of all Pfizer trial participants worldwide.
Complete information about adverse events during this extensive trial in Argentina does not appear to have been released as of this writing.
However, Roux’s experience has since become public.
Roux, often referred to as the “disappeared” patient, volunteered for the trial (volunteer number 12312982) and received his first dose of the Pfizer vaccine on Aug. 21, 2020.
According to Healy, Roux “felt pain and swelling in his arm right after the injection. Later that day he had nausea, difficulty swallowing, and felt hungover.”
After a series of symptoms, Roux — during a clinical trial visit on Aug. 23, 2020 — was classified as experiencing a “toxicity grade 1 adverse effect.”
He nevertheless received his second dose on Sept. 9, 2020.
According to Healy:
“On the way home by taxi, he started feeling unwell. At 19:30, he was short of breath, had a burning pain in his chest and was extremely fatigued. He lay on his bed and fell asleep. He woke up at 21:00 with nausea and fever (38-39 C) and was unable to get out of bed due to the fatigue.
“Over the next two days, he reports a high fever (41 C) and feeling delirious.
“On September 11, he was able to get out of bed and go to the bathroom when he observed his urine to be dark (like Coca-Cola). He felt as if his heart expanded, had a sudden lack of breath and fell unconscious on the floor for approximately 3 hours.
“Once he recovered, he felt tired, was uncomfortable, had a high heart rate on minor movement, was dizzy when changing posture. He had a chest pain which radiated to his left arm and back.”
On Sept. 12, 2020, Roux was admitted to the Hospital Alemán, where he stayed for two days. It was initially believed he had COVID-19, but he tested negative for the virus. His symptoms also were found to not correspond with viral pneumonia.
After a series of X-rays, CT scans and urine tests, Roux was discharged Sept. 14, 2020, after being diagnosed with an adverse reaction — specifically, an unequivocal pericardial effusion — to the coronavirus vaccine (high probability), according to his discharge summary.
Doctor who altered Roux’s record had ties to Gates, NIH, Big Pharma
However, on Sept. 17, Dr. Fernando Polack, Pfizer’s lead investigator for the Argentine trials according to a Pfizer document released in December 2021, reported in Roux’s record that his “hospitalization was not related to the vaccine.”
Even after Roux’s discharge, his health difficulties continued. As reported by Healy:
“On November 13 [2020], he had negative IgG and IgM SARS COV-2 (QML technique), which is unusual post vaccine.
“On February 24, 2021, a liver scan showed a minor degree of abnormality. In March 2021 and February 2022, his liver enzymes remained abnormal.”
Ultimately, Roux lost 14 kilograms (30.8 pounds) in a period of three to four months, and continued to suffer from fever and bouts of breathlessness for several months afterward.
Polack, who reported Roux’s hospitalization as unrelated to the vaccination, is known for his close ties with various vaccine manufacturers, pharmaceutical companies and the Bill & Melinda Gates Foundation.
For instance, he is listed as the lead author in a Dec. 31, 2020, New England Journal of Medicine (NEJM) article on the purported efficacy of the Pfizer COVID-19 vaccine.
According to Healy, Polack also appears to be the founder of iTRIALS, a trial site management company, and another organization located at the same physical headquarters, the Fundación INFANT.
Healy wrote:
“When COVID struck Argentina, [Polack] and his Fundación became involved in a trial of immune plasma, taken from patients who had recovered from COVID, given to patients who had recently acquired the disease.
“In May 2020 he speculated that this would make COVID like an ordinary cold, and the Gates Foundation would offer financial support. He used high-profile press conferences to disseminate his exciting message.”
The conclusion of the study published in the NEJM following the plasma study reads:
“Funded by the Bill and Melinda Gates Foundation and the Fundación INFANT Pandemic Fund; Dirección de Sangre y Medicina Transfusional del Ministerio de Salud number, PAEPCC19, Plataforma de Registro Informatizado de Investigaciones en Salud number, 1421, and ClinicalTrials.gov number, NCT04479163.”
According to Healy, “[a] subsequent systematic review and meta-analysis failed to confirm these findings, noting ‘very serious imprecision concerns.’”
Healy pointed out that Polack, in his NEJM disclosure statement, did not indicate any conflict of interest or financial interest in the COVID-19 vaccine trials in Argentina, but:
“Polack reported grants from Novavax and personal fees from Janssen, Bavarian Nordic A/S, Pfizer, Sanofi, Regeneron, Merck, Medimmune, Vir Bio[technology], Ark Bio, Daiichi Sankyo outside the submitted work.
“At least eight of these companies are engaged in RSV vaccine research in babies and pregnant women. Fernando has mentioned a combined RSV, flu and COVID vaccine.”
And, in relation to Polack’s relationship with the Bill & Melinda Gates Foundation, Healy reported:
“[Polack] also doesn’t mention his extensive financial involvement with the Bill & Melinda Gates Foundation. This organization supports industry vaccine trials including Covid and RSV. Fernando is heavily involved through his Gates-sponsored Fundación INFANT in Buenos Aires in RSV trials and research.
“Gates sunk $82,553,834 into Novavax’s RSV vaccine ResVax which was shown to be ineffective in clinical trials in pregnant women.”
Polack’s own bio from a 2017 medical conference states “[h]is work is funded by the Bill & Melinda Gates Foundation, the National Institutes of Health [NIH], the Thrasher Research Fund, the Optimus Foundation and other international organizations.”
That same year, Polack testified at an FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting, where he “acknowledged having financial interests in or professional relationships with some of the affected firms identified for this meeting, namely Janssen [producer of the Johnson & Johnson COVID vaccine], Novavax, and Bavarian Nordic.”
According to Dr. Joseph Mercola, Polack “also happens to be a consultant for the U.S. Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC),” and “a current adjunct professor at Vanderbilt University in Tennessee.”
Michael Nevradakis, Ph.D., is an independent journalist and researcher based in Athens, Greece.



